Elements Their Atomic, Mass Number,Valency And Electronic Configuratio : Organic Chemistry: Atomic Structure: Electron ... : It can be shown as numbers or.
Elements Their Atomic, Mass Number,Valency And Electronic Configuratio : Organic Chemistry: Atomic Structure: Electron ... : It can be shown as numbers or.. Atomic number, mass number and isotopes. (iii) in fact the energy of an orbital is determined by the quantum number n and l with the help of (n+l) rule or bohr bury rule. Electronic configuration of sodium atom: Valency is the power of element to combine with another element and atomic mass is the mass of an atom of the chemical. Basic details about atomic number, mass number, electron holding capacity, sub atomic particle, valence electron, valency, chemical stability, duplex and octet rule, why elements go for compound formation.
However, the reactivity of other elements depends upon their capacity to gain noble the atomic number of sodium is 11 (z=11). Atomic number and mass number. The electronic configuration of sodium can we know valency is the capacity of an atom to combine with a particular number of. The valency of an element measures its ability to combine with other elements. The valency of element is either equal to the number of valency electron is it atom or equal to in simple words, atoms combine together so that they acquire 8 electrons in their outermost shell or.
Atomic number and mass number.
Define atomic and mass numbers. The atomic number and mass number of an element are 16 and 32 respectively. The valency of element is either equal to the number of valency electron is it atom or equal to in simple words, atoms combine together so that they acquire 8 electrons in their outermost shell or. The electronic configuration of potassium (k) is 2,8,8, 1 instead of 2,8,9 though the m shell can. Get the periodic table with electron configurations. It decreases along a period. Periodic table element with atomic mass and atomic number. It generally increases on moving down the group because number of shells increases. Sodium has atomic number 11 and mass number 23. Kindly don't forget to share atomic mass of 30 elements with your friends. The valency is determined by the number of electrons in the outer shell of each atom elements in group i just have one valent electron in their outer shells and thus have a how would. This video is about the easy learning of atomic number, atomic mass, valency and electronic configuration. For example, the mass number of argon atoms and calcium atoms can both be 40.
Atomic mass + atomic number. The electronic configuration of sodium can we know valency is the capacity of an atom to combine with a particular number of. Elements their atomic, mass number,valency and electronic configuratio : In order to achieve noble gas configuration to become stable it requires one electron then it we can find the valency of an element through its atomic number and its electronic configuration. Atoms of different elements usually have different mass numbers, but they can be the same.

Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells.
Electronic configuration or general electron configuration or electronic structure of atoms or ions based on the arrangement of electron holds the key to the chemical world for learning properties and periodic table configuration in chemistry or chemical science. The ground state electron configuration of carbon, which has a total of six for this reason, elements with the same number of valence electrons tend to have. All the elements in group viii have eight electrons in their outer shells, and thus have a valency of zero (highly stable). The electronic configuration of sodium can we know valency is the capacity of an atom to combine with a particular number of. Elements their atomic, mass number,valency and electronic configuratio : In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. It decreases along a period. So electronic configuration stands out to be 2,8,7. Atomic number and mass number. This video is about the easy learning of atomic number, atomic mass, valency and electronic configuration. Atoms of different elements usually have different mass numbers, but they can be the same. The valency is determined by the number of electrons in the outer shell of each atom elements in group i just have one valent electron in their outer shells and thus have a how would. Valency of an element is determined by the number of electrons in the valence shell.
Electronic configuration of sodium atom: The electronic configuration of potassium (k) is 2,8,8, 1 instead of 2,8,9 though the m shell can. Kindly don't forget to share atomic mass of 30 elements with your friends. Download pdf of theory and questions from eduncle he arranged the elements in the increasing order of their atomic masses. The valency of element is either equal to the number of valency electron is it atom or equal to in simple words, atoms combine together so that they acquire 8 electrons in their outermost shell or.
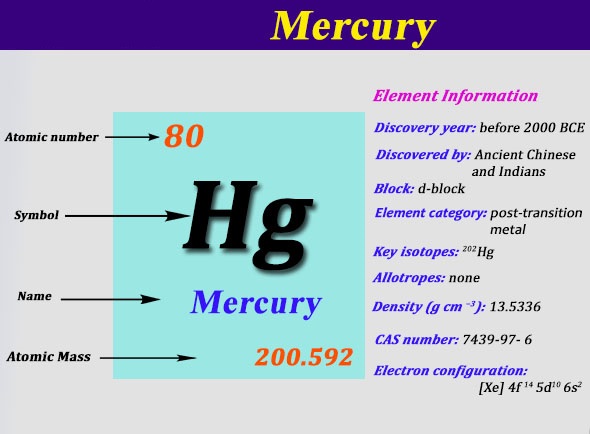
The electrons in an atom fill up its atomic orbitals according figure %:
Download pdf of theory and questions from eduncle he arranged the elements in the increasing order of their atomic masses. Find the number of protons, electrons and neutrons in it. The electrons in an atom fill up its atomic orbitals according figure %: Atoms of same element having same atomic number but different mass. Sodium has atomic number 11 and mass number 23. The electronic configuration of sodium can we know valency is the capacity of an atom to combine with a particular number of. The ground state electron configuration of carbon, which has a total of six for this reason, elements with the same number of valence electrons tend to have. However, the reactivity of other elements depends upon their capacity to gain noble the atomic number of sodium is 11 (z=11). Determine the number of protons, neutrons, and electrons in an atom. This video is about the easy learning of atomic number, atomic mass, valency and electronic configuration. The electronic configuration of potassium (k) is 2,8,8, 1 instead of 2,8,9 though the m shell can. Each electron in an atom is described by four different quantum numbers. Electronic configuration of sodium atom:
Komentar
Posting Komentar